10 Unusual Facts About Iron That Will Surprise You
Iron is everywhere—inside your blood, in the steel that builds cities, even deep in the core of our planet. Yet most people know very little about this extraordinary element. Far beyond “iron is strong” or “iron rusts,” there are unusual facts about iron that reveal just how weird and fascinating it really is.
Here are 10 of the most surprising truths about iron that will make you see this common metal in a whole new way.
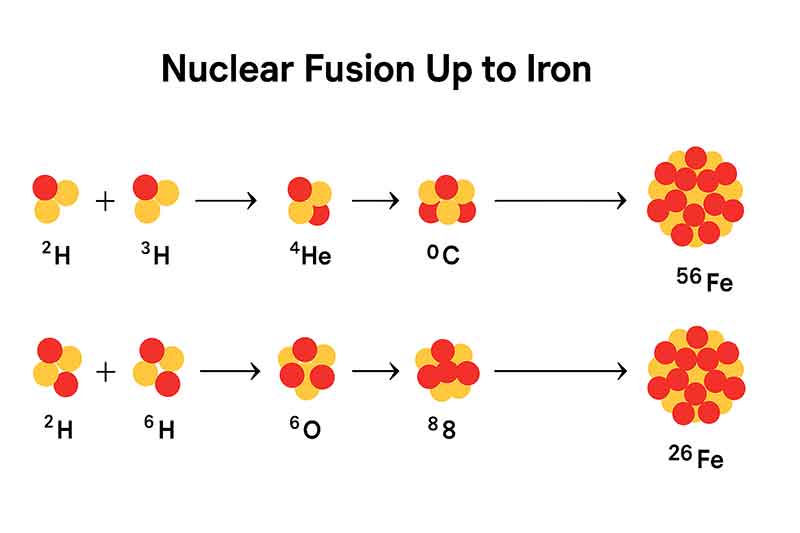
1. Iron Is the Final Step in Star Fusion
Stars fuse lighter elements into heavier ones, but the process stops at iron. Unlike lighter elements, iron cannot release energy through fusion. This makes iron the “dead-end” of stellar life—and the reason massive stars explode into supernovae when iron builds up in their cores.
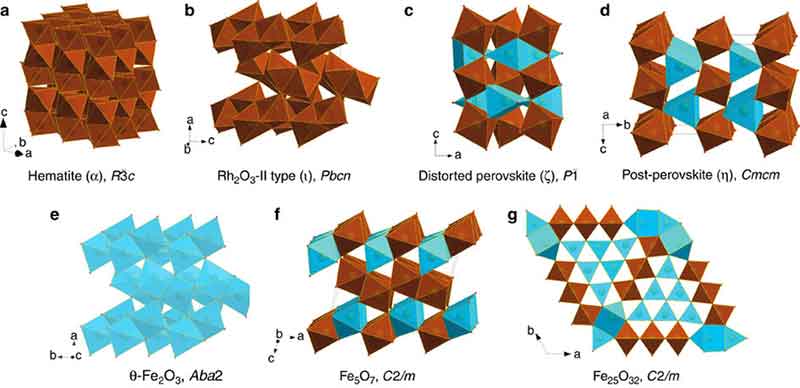
2. Earth’s Core Isn’t Pure Iron
Deep inside Earth, iron exists under pressures and temperatures so extreme that it changes its crystal form. Scientists discovered that instead of being uniform, the core contains a mix of phases—body-centered cubic (bcc) and hexagonal close-packed (hcp). This explains strange behaviors like seismic wave variations traveling through the planet.
3. Iron Can Be Magnetic—or Not, Depending on Its Form
At room temperature, iron is ferromagnetic, which means it sticks to magnets. But in its high-temperature gamma phase (austenite), iron actually becomes non-magnetic. Even pure iron can suddenly stop being magnetic, depending on its crystal structure and temperature.
4. Mars Is Red Because of Iron
Mars owes its signature rusty color to iron oxide dust covering the planet’s surface. Without iron, the “Red Planet” would look very different. Iron literally paints the solar system.
5. Meteorites Gave Us the First Iron Tools
Long before humans learned how to smelt ore, ancient cultures crafted weapons and jewelry from iron meteorites. These tools were more valuable than gold, since they came from the sky. In fact, the word “iron” in many ancient languages is linked to “heaven” or “sky metal.”
6. Iron in Your Body Is Both Vital and Dangerous
You need iron to survive—it allows red blood cells to carry oxygen. But free iron floating in your body is highly toxic. That’s why proteins like ferritin and transferrin tightly bind and transport it. Too little iron causes anemia; too much damages your organs.
7. Iron Has Unusual Compounds With Wild Properties
Iron doesn’t just form rust. For example:
- Carbonyl Iron: a highly pure powder used in electronics and even radar-absorbing coatings.
- Iron Tetraboride (FeB₄): a superhard material that also becomes superconducting at very low temperatures—two properties rarely found together.
8. Iron Exists in Rare Exotic Oxidation States
Most people know iron as Fe²⁺ or Fe³⁺. But under lab conditions, chemists have created unusual compounds with oxidation states as high as +6 or +7. These exotic states reveal how versatile iron can be in chemical bonding.
9. Iron Shapes Civilizations
The Iron Age transformed human history. Stronger than bronze, iron tools and weapons allowed societies to grow faster, build stronger, and fight harder. Ancient furnaces in Africa, India, and the Middle East show that advanced ironworking began earlier than many history books suggest.
10. Iron Is Rare in Its Pure Metallic Form on Earth’s Surface
Even though iron makes up about 35% of Earth by mass, you won’t find shiny iron chunks lying around. Pure iron reacts quickly with oxygen and water, forming oxides. That’s why metallic iron on the surface usually comes from meteorites, not the planet itself.
From powering stars to coloring planets, from shaping civilizations to keeping you alive, iron is one of the most unusual and important elements in the universe. Next time you see a rusty nail or a skyscraper, remember—you’re looking at a metal that tells the story of both cosmic explosions and human progress.
Related Facts:







